Research contents
- 1. Chain reaction from innate to acquired immunity ~Immunological mechanism of artificial adjuvant vector cells~
- 2. Elucidating memory immunity
- 3. The immunotherapeutic strategy using tumor-derived neoantigens
- 4. Infectious Disease Project
1. Chain reaction from innate to acquired immunity
~Immunological mechanism of artificial adjuvant vector cells~
Our basic research focusses on the chain reaction of immune response, involving the mechanism of induction initiated by triggering the immune response cascade by innate immunity (mainly the mutual activity of lymphocytes and dendritic cells) to acquired and memory immunity. In addition, the focus is also on translational research for immunotherapy using this mechanism. In particular, to artificially and efficiently activate the immune system, we have designed and are developing artificial adjuvant vector cells (aAVC), which can maximally utilize the functions of dendritic cells existing in vivo.
1.1. The immunological interrelationship between natural killer T cells and dendritic cells
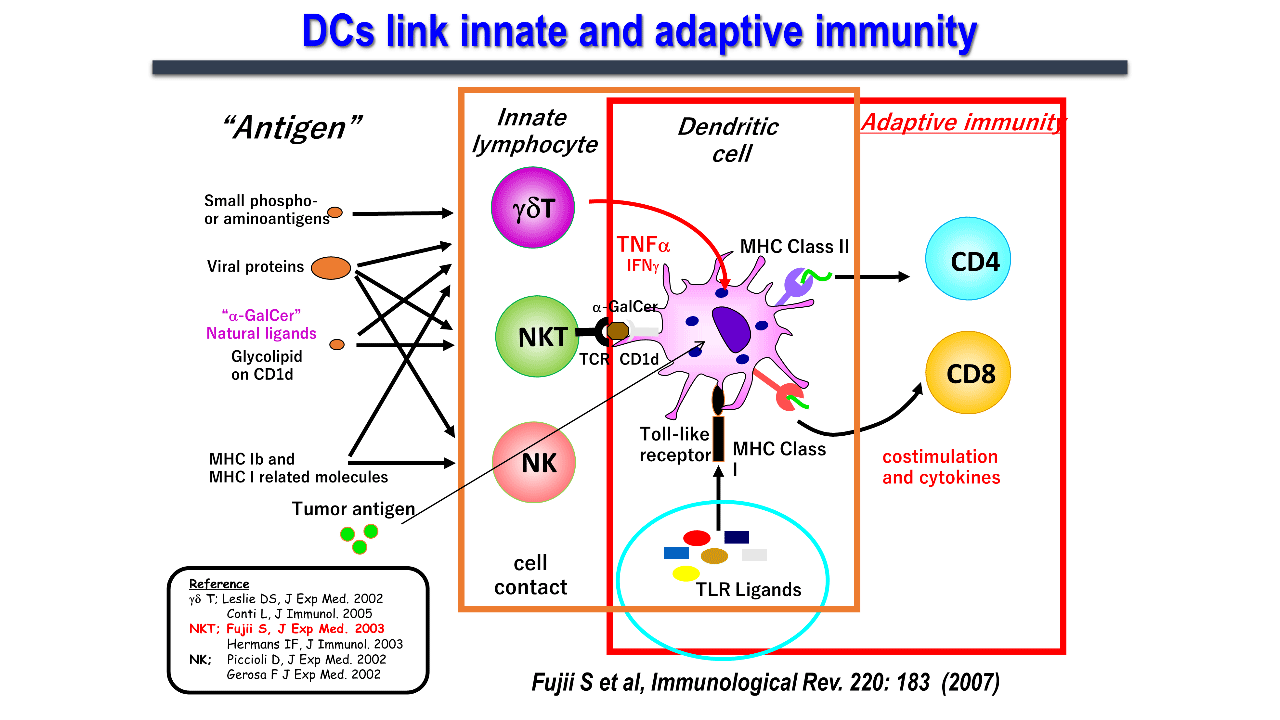
Dendritic cells, which link innate and acquired immunity, induce antigen-specific immunity through activation and maturation. Toll-like signaling has been studied as a maturation signal. However, in 2003, we first reported a novel phenomenon wherein, natural killer T (NKT) cells induced dendritic cell maturation through cell-cell interactions (Figure 2)..
Invariant NKT (iNKT) cells are a type of innate immune system which express receptors for both T and natural killer (NK) cells. They are mainly found in organs such as the liver, spleen, lungs, and bone marrow, and are less abundant in the lymph nodes. iNKT cells, commonly known as NKT cells, which express an invariant chain (Vα14Jα18 in mice and Vα24Jα18 in humans) of the T-cell receptor (TCR) without a variable α-chain, can recognize sugar glycolipid antigens presented on CD1d molecules. The CD1d molecule is identical among humans, and is considerably homologous between mice and humans. iNKT cells from both human and mice recognize CD1d/α-GalCer complex and activate. iNKT cell activation in vivo leads to dendritic cell maturation as shown in the figure and activates multiple immune cells. Human iNKT cells also elicit similar immune responses in vitro and in immunodeficient mouse models. Thus, the research using this ligand on mouse models can be used for human clinical applications.
1.2. Immune mechanism of artificial adjuvant vector cells
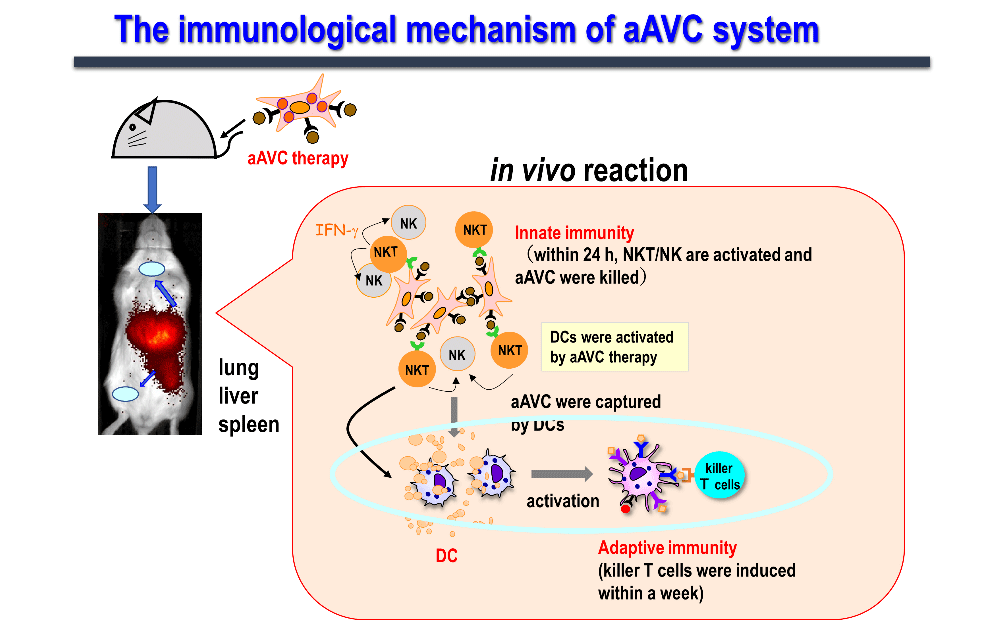
Cancer vaccines can be used for cancer treatment, as well as to prevent recurrence in patients resistant to surgery, chemotherapy, and radiotherapy. However, each method has its own limitations. For example, dendritic cell therapy encounters difficulties in efficient dendritic cell maturation, collecting patient-derived dendritic cells, and the cell culture process. Moreover, the high medical costs associated with the cell culture process are also limiting. Peptide vaccine therapy is limited to a small group of patients because of its human leukocyte antigen (HLA)-binding nature. Furthermore, an induction of antigen-specific CD8+ T cells by peptide therapy is insufficient for clinical efficacy. Therefore, our laboratory has been conducting active research to develop new therapeutic cancer vaccines which can address the drawbacks associated with conventional cancer vaccines, such as inducing innate, acquired, and even memory immunity. Although this approach represents applied research, it is conceptually based on basic research. The concept of aAVC has also originated from our basic research on the interaction between activated NKT cells and dendritic cells in vivo, as described earlier.
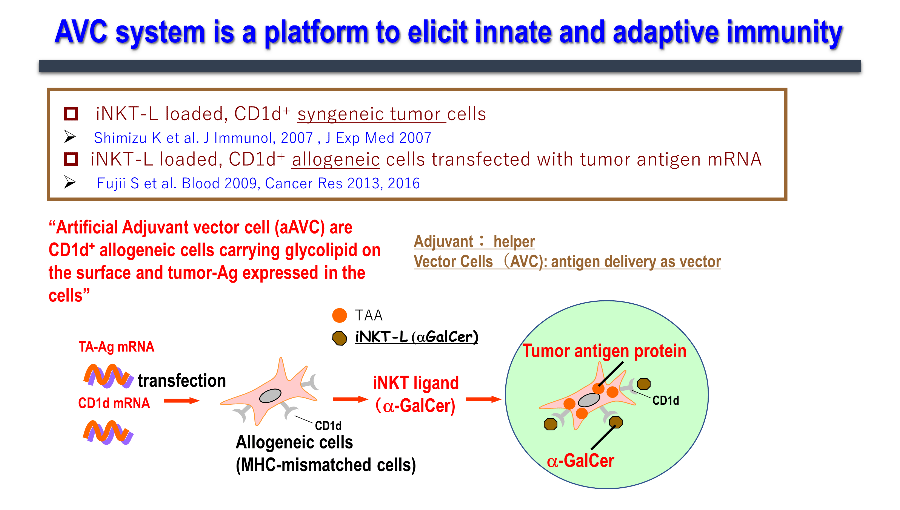
We have been working on developing a cancer vaccine that explores the immune mechanism wherein activated NKT cells induce the maturation of dendritic cells and acquired immunity. We have realized that simultaneous expression of the tumor antigen and NKT ligand (α-GalCer) on CD1d-expressing cells is the most efficient strategy to activate innate lymphocytes, such as NK, iNKT, and antigen-specific T cells, thus exhibiting antitumor effects. Cells expressing these two antigens are "vector" cells carrying target antigens (protein and glycolipid antigens) simultaneously to dendritic cells in situ, and are termed "adjuvant vector cells", owing to their function of "adjuvant" cells which activate dendritic cells in situ. The simplest strategy is to use the cell itself as an adjuvant vector cell. The simplest model is the autologous adjuvant vector cell model (i.e., tumor/Gal), wherein the tumor cells themselves express the tumor antigen and CD1d/α-GalCer complex. Next, we developed aAVC as a cellular drug. aAVC is a completely new cellular drug in which mRNA of CD1d molecule and mRNA derived from cancer (tumor-associated antigen: TAA) or viral antigens are co-introduced into allogeneic cells, and loaded with NKT cell ligand (α-GalCer), resulting in the expression of α-GalCer/CD1d complex on the cell surface and cancer or viral antigen protein inside the cells (Figure 3). The "aAVC" is an off-the-shelf improved model which can be generally applied by using third party cells. Regarding immune mechanism, vaccines using these two types of adjuvant vector cells derived from autologous cells or from other cells can be used as in vivo dendritic cell-targeted therapies, since they maximize the function of dendritic cells in vivo (Figure 4).
1.3. Applications of aAVC
The aAVC system serves as a platform technology. Since the target antigens of aAVC are interchangeable, antigen-specific T cell responses have been verified using various antigens, such as model, cancer, and viral antigens (including OVA, WT1, Trp2, MART-1, NY-ESO-1, Influenza HA, and SARS-CoV-2 spike). We are currently developing aAVC expressing various cancers and viral antigens.
2. Elucidating memory immunity
2.1 Memory system of innate immunity (long term effector iNKT cells)
Although NK, γδT, and iNKT cells (known as innate lymphocytes) are presumed to act immunologically quickly, a memory immune subset for each of them has recently been identified, wherein cells of the innate immune system also respond to antigens.
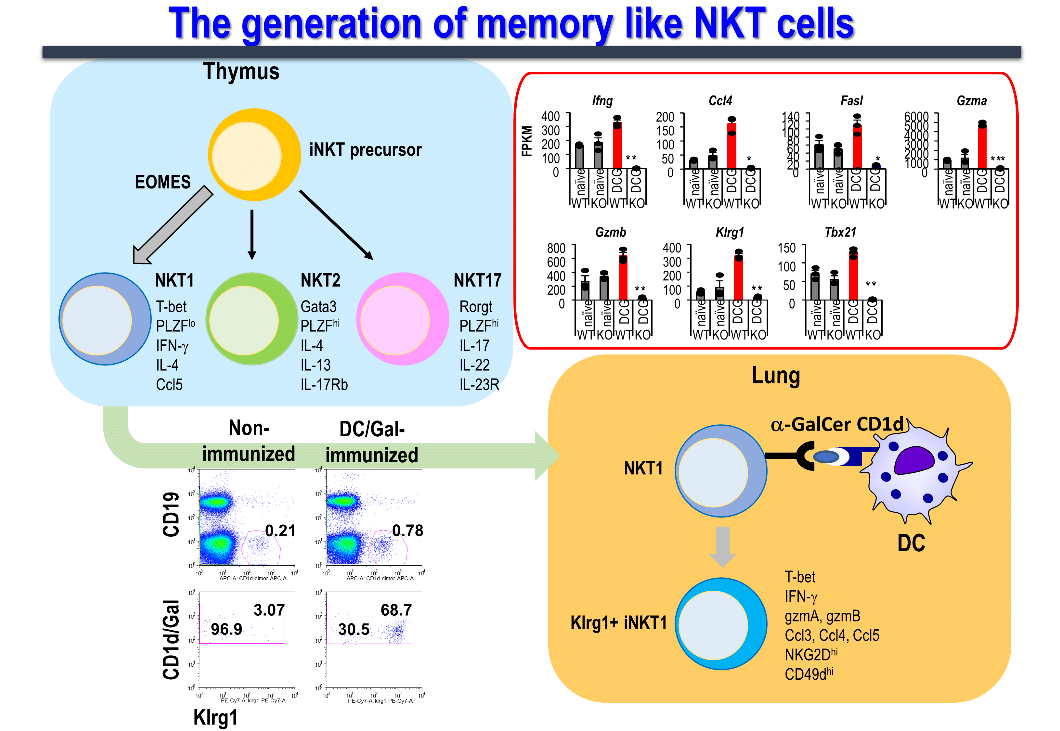
In our laboratory, we investigated in detail the duration of the effector function of NKT cells in mice after the administration of dendritic cells pulsed with α-GalCer (DC/Gal), and determined the presence of memory cells in iNKT cells ,(Figure 5). As a result, we identified a subset of long-term effector iNKT cells that survived for more than 9 months after sensitization stimulation and responded rapidly and strongly to a second stimulation. Moreover, since memory iNKT cell induction can be detected similarly by "Allogeneic fibroblasts expressing CD1d and presenting α-GalCer," which was used to create the above-mentioned aAVC, it was confirmed that the immune response is glycolipid antigen-dependent, and independent of dendritic cell-derived molecules.。
Memory iNKT cells express killer cell lectin-like receptor subfamily G member 1 (Klrg1) on the cell surface, produce high levels of the cytokine, interferon-γ (possessing antitumor effects), and TCR repertoire analysis of memory iNKT cells revealed the accumulation of specific clones. Furthermore, the anti-tumor activity of memory iNKT cells was verified in a lung metastasis model of B16 malignant melanoma in mice intravenously administered 4 months after DC/Gal immunization, showing suppression of melanoma metastasis. We have confirmed that this anti-tumor effect is mainly due to the involvement of memory iNKT cells. This novel finding shows that iNKT cells, which were assumed to be innate immune lymphocytes, also have characteristic memory iNKT cells. Currently, our research focuses on elucidating the molecular mechanisms underlying iNKT cell memorization.
2.2 Generation and maintenance of memory cytotoxic T cells
We are conducting studies in humans and mice to elucidate the factors defining memory T cell induction, memory T cell subsets, and functions. Generally, vaccine efficacy not only involves the temporary induction of effector cells, but also the long-term induction of memory T cells, which is important to prevent tumor recurrence. When aAVC-expressing OVA antigen (aAVC-OVA) was administered to mice and analyzed after 1 year, we found that memory killer T cells were maintained systemically in lymphoid (the lymph nodes, spleen, and bone marrow) as well as non-lymphoid tissues (the lungs and liver) (Figure 6). These memory T-cells can be robustly amplified on encountering the same antigen.
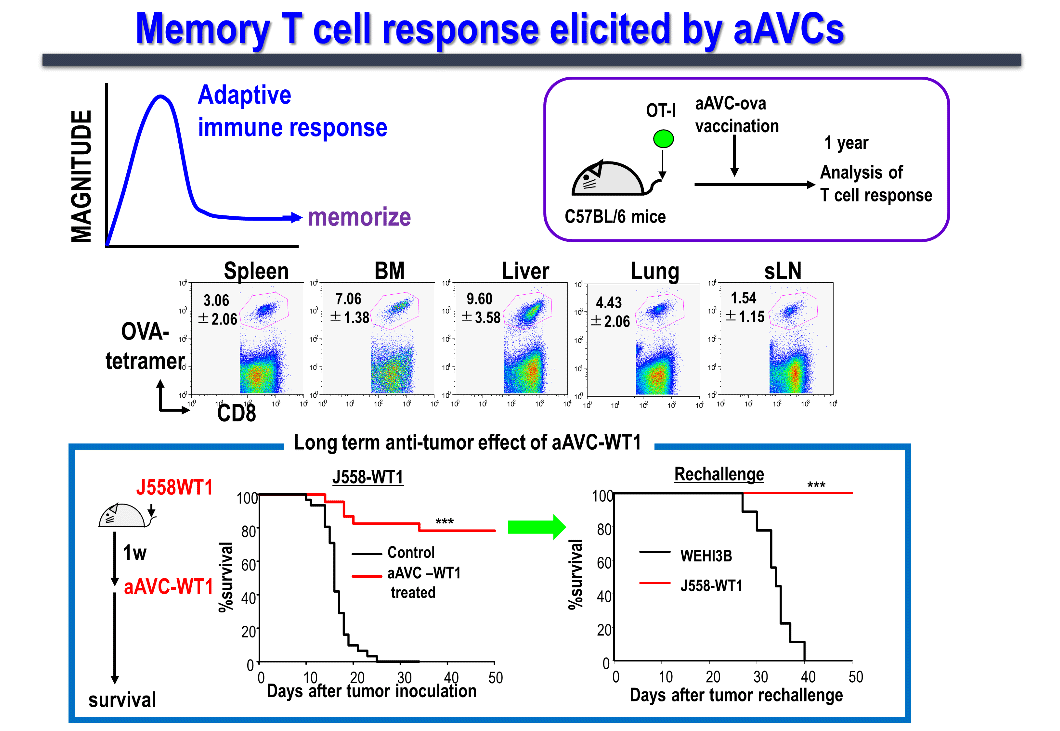
The first-in-human of Phase 1 investigator-initiated clinical trial of the whole WT-1 antigen-expressing aAVC (aAVC-WT1) for the patients with relapsed and refractory acute myeloid leukemia (RR-AML) has completed. We confirmed that, both, CD4+ and CD8+ T cells responded to the peptide library, proliferated, and persisted for up to 12 months as long-term memory killer T cells. Furthermore, single-cell transcriptome and TCR analyses at the tumor site (bone marrow) verified the possibility of tracking at the clone level. In addition, we were able to obtain a 50% or more reduction in tumor volume (leukemia cells) in about half of the cases, CRi (complete remission with incomplete hematologic recovery) in three cases as Proof of concept (POC) of a certain clinical efficacy.
3. The immunotherapeutic strategy using tumor-derived neoantigens
3.1 Prediction system of neoantigens for efficient immune induction
In patients with cancer, the clinical utility of anti-tumor cytotoxic T cells, which are reactivated or enhanced by immune checkpoint blockade therapy, is well-known. Compared with self-antigens, neoantigens derived from tumor somatic mutations are ideal immune targets in tumors. The tumor cell-derived neoantigens can be identified using immunogenomic or immunopeptide approaches as a predictive system.
3.2 The identification of neoantigens has highlighted several Programmed death-ligand 1 expression-neoantigen associations
Our research team aimed to elucidate the association between programmed death-ligand 1 (PD-L1) expression in cancer tissues and immune responses targeting neoantigens as predictors of the response to immune checkpoint inhibitors. Although programmed cell death protein-1 expressed by tumor sites is a predictive marker of immune checkpoint inhibitor response, it cannot be universally applied to all patients. To rationalize this, we used a mouse model and found that low PD-L1 expression enhances the immune response to neoantigens. Furthermore, a dendritic cell vaccine using the identified neoantigen mixture induced a neoantigen-specific T-cell response and had a therapeutic effect comparable to that of immune checkpoint molecules.
4. Infectious Disease Project
4.1 Cross-reactive T cell research in coronavirus disease
In infectious diseases, the presence or absence of memory immunity to the pathogen determines the prevalence of the disease in individuals and populations. Cross-responsiveness of memory immunity may potentially protect against infection, provided it is not a re-infection with the exact same pathogen, but with the same virus species or strain. Human coronaviruses, including SARS-CoV-2, the causative agent of coronavirus disease (COVID-19), regularly cause outbreaks of zoonotic diseases characterized by severe pneumonia, such as the severe acute respiratory syndrome (2002) and Middle East respiratory syndrome (2012). Antiviral immunity is primarily attributed to neutralizing antibodies and cytotoxic T cells (CD8+ T cells). Neutralizing antibodies can prevent viral infection by recognizing and binding to the virus, whereas CD8+ T cells recognize viral epitopes on major histocompatibility complex (MHC) class I of infected cells, attack them, and eliminate the virus. To understand the relationship between HLA alleles and COVID-19 in the immune response to SARS-CoV-2, our research team analyzed the frequency of the following one of major HLA population: the type common in Japanese individuals (HLA-A*24:02). According to the World Health Organization database, the number of COVID-19-related deaths per 100,000 population in each country was compared with the frequency of HLA-A*24:02 positive population, and it was found that the HLA-A*24:02 positive rate was inversely correlated with the number of COVID-19-related deaths per 100,000 population. Thus, HLA class I may reflect the immune response of CD8+ T cells; we focused on HLA-A*24:02 to study cross-reactive T cells.
Among the epitopes with high affinity for HLA-A*24:02, we identified a cross-reactive epitope, S1208-1216 (QYIKWPWYI; QYI), which is highly homologous to seasonal coronaviruses. Most of HLA-A*24:02 positive healthy individuals possess CD8+ T cells that are cross-reactive to this QYI peptide, which is consistent with the fact that memory CD8+ T cells induced by previous seasonal coronavirus infections. In addition, we verified cross-reactivity at the TCR level. We have also reported that cross-reactivity can be explained by the similarity in the crystal structure of peptide-HLA in collaboration with Dr. Shirouzu, a team leader of the Functional and Structural Research Team, RIKEN BDR.
4.2 Vaccine Development Research against Infectious Diseases
We are developing vaccines against infectious diseases based on the use of aAVC. Through animal experiments, we have established that, in addition to cytotoxic T-lymphocytes (CTL), aAVC-expressing influenza hemagglutinin (HA) antigen (aAVC-HA) induces the production of HA antigen-specific antibodies via antigen-specific CD4Tfh. Its protective efficacy against infection has also been demonstrated using an influenza infection model. We also applied an aAVC system to target the SARS-CoV-2 spike protein (CoV-2-S) using CoV-2-S-expressing aAVCs (aAVC-CoV-2) and evaluated the immune response in a murine model. A single dose of aAVC-CoV-2 induced a large number of CoV-2-S-specific multifunctional CTLs, in addition to CD4+ T cell-dependent anti-CoV-2-S-specific antibodies. In addition, we ensured that a single immunization with a cellular vaccine conferred dual protection against SARS-CoV-2 and cancer. To this end, we immunized mice with CoV-2-S- and tumor-associated antigen (TAA)-co-expressing aAVCs (aAVC-TAA/CoV-2) and evaluated whether anti-SARS-CoV-2 and antitumor CTLs were elicited. We found that aAVC-TAA/CoV-2-S therapy exerted apparent antitumor effects and induced CoV-2-S-specific CTLs lymphocytes too. These findings suggest that aAVC-TAA/CoV-2-S therapy is a promising vaccine candidate for preventing COVID-19 and enhancing the effectiveness of cancer therapies. This is intended to be developed as a next-generation vaccine against COVID-19 for high-risk groups, such as patients with cancers.
